Different compounds may have very different absorption maxima and absorbances. You may repeat.

Uv Vis Spectrophotometry Easy And Quick Quantification Of Nucleic Acids Eppendorf Handling Solutions

Why Is It Advisable To Use The Maximal Wavelength For An Absorbance Peak When Measuring The Absorbance To Determine The Concentration Of A Compound Quora
1
Select one that has Absorbance.
Maximum absorbance value. In a microplate reader the same DNA concentration measured will lead to a smaller OD value about 07 OD because of the smaller path length in a microplate well. This wavelength will be used to monitor the disappearance of crystal violet throughout the course of the experiment. This is a measure of how easily light passes through a material.
The main absorbance equation is the Beer-Lambert Law. The values labeled on the X axis are the bin centers. Therefore if Ê is known measurement of A gives the concentration directly e is normally quoted for a 1-cm path length.
Integrated absorbance not peak absorbance depends linearly on concentration. This will enable you to plot a graph of Velocity of reaction absorbance units per sec against Substrate concentration M. Intensely absorbing compounds must be examined in dilute solution so that significant light energy is received by the detector and this requires the use of completely transparent non-absorbing solvents.
The Bradford assay is a colorimetric assay that measures protein concentration. Because of low sensitivity. Short answerUsing the maximum wavelength gives us the best results.
Maximum absorbance number exceeds 10 dilute the sample by removing half the volume of the dye and replacing it with an equal amount of methanol. 100 Absorbance 0 Transmittance While a spectrophotometer can display measurements as either transmittance or absorbance in biological applications we are usually interested in the absorbance of a given sample. Record both λ max and the absorbance values of all the samples.
Experiment was 1 cm the value of the molar absorptivity was equal to the slope of the line. Maximum absorbance is observed. Measure the absorbance at 450 nm using a microplate.
This is because at the peak absorbance the absobance strength of light will be. The wavelength of maximum absorbance is a characteristic value designated as λ max. Determined value of ε.
Value of a blank solution from the absorption value of each well. A rule of thumb is to start with a number of bins that is equal to the square root of the largest value in your data sets to be plotted. The numerical integration of the absorbance leads to maximum deviations from linearity of less than 01.
In this example bin 10 m contains values that range from 950 -1049 m. Thus it was determined that the molar absorptivity of the CuSO 4 at 635 nm was 281 M-1cm. Unlike optical absorbance the absorbance coefficient represents the optical attenuation per unit length of material typical units are 1cm.
Indeed the entire vertical absorbance scale may be changed to a molar absorptivity scale once this information about the sample is in. From the graph find the maximum velocity and half it ie. The reagent Coomassie brilliant blue turns blue when it binds to arginine and aromatic amino acids present in proteins thus increasing the absorbance of the sample.
This procedure NEEDS to be repeated at EACH wavelength. Absorbance is different for EVERY chemical at EVERY wavelength so every time you change. Sample size is clearly indicated either.
The concentration dependence of absorbance can deviate from linearity even in the absence of any interactions or instrumental nonlinearities. Plot the absorbance vs. - Look at the maximum absorbance in your graph - Look at the minimum absorbance in your.
Wavelength and connect the points to from a smooth curve. Repeat the dilution process until the maximum absorbance is below 1. If a 24-well or 6-well plate is used for this assay.
Spectrophotometry uses photometers known as spectrophotometers that can measure the intensity of a light beam at different wavelengthsAlthough spectrophotometry is most commonly applied to. Let us take a compound with a very high value of molar absorptivity say 100000 L mol-1 cm-1 which is in a solution in a 1 cm path-length cuvette and gives an absorbance of 1. Tecans Sunrise absorbance microplate reader offers all the functionality needed for numerous photometric applications including advanced 12-channel optics for fast multichannel absorbance reading of ELISAs a temperature control function for enzyme kinetic assays and a tunable wavelength function for wavelength scanning.
Once you have reached 600 nm return to the region where absorbance was maximum and collect absorbance readings at 5 nm intervals in this region. The absorbance value is plotted on the vertical y-axis against the wavelength of light used for a given test plotted on the horizontal x-axis. After performing an absorbance measurement the result is a value given in either transmission or optical density.
E 1 1 c. This wavelength is known as. Whereas quantification of a substance in solution is the goal of the measurement then the obvious question is how to convert the known signal into the concentration value.
Select the proper wavelength to use for the determination of iron with 110-phenanthroline and calculate the. A Absorbance Êcl where Ê extinction coefficient c concentration in molL and l optical path length in cm. The recommended maximum number of cells per well for the 96-well plate is 25000.
Plotting the maximum absorbance values for each wavelength of light tested produces the samples absorbance spectrum and identifies the compounds making up the test substance and their proportions. Therefore c 1 100000 1 10-5 mol L-1. Draw a horizontal line from this point till you find the point on the graph that corresponds to it and read off the substrate concentration at that point.
Here we use 310nm as the wavelength for absorbance. Based on the preliminary studies we confirmed that at this wavelength maximum aggregation occurs ie maximum absorbance. Spectrophotometry Page 2 of 10 solution Transmittance or absorbed Absorbance by the solution is measured by a light meter.
Therefore with a conventional absorbance reading an A260 of 10 OD corresponds to 50 μgml dsDNA solution. The absorbance is measured using a spectrophotometer at the maximum absorbance frequency A max of the blue dye which is 595 nm. Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength.
With the optional low volume NanoQuant Plate and cuvette port this reader represents a perfect tool for DNARNA quantifications A 260280 and purity checks A. Reread the maximum absorbance. However the goal of the measurement is the quantification of a substance in solution the obvious question is how to convert the signal into the concentration value.
Exceptional wavelenth accuracy typically. After the absorbance measurement the result is a value given in either transmission or optical density. If the isoprene spectrum on the right was obtained from a dilute hexane solution c 4 10-5 moles per liter in a 1 cm sample cuvette a simple calculation using the above formula indicates a molar absorptivity of 20000 at the maximum absorption wavelength.
Higher absorbance coefficients indicate that lower amounts of incident light will pass through the material. Record the absorbance value that it gives you. CV A 1 cmε 3 The first step in this analysis will be to determine the wavelength of maximum absorbance for crystal violet and the corresponding molar absorption coefficient at that wavelength.
The value obtained will depend on the path length of the cuvet.
Assets Thermofisher Com
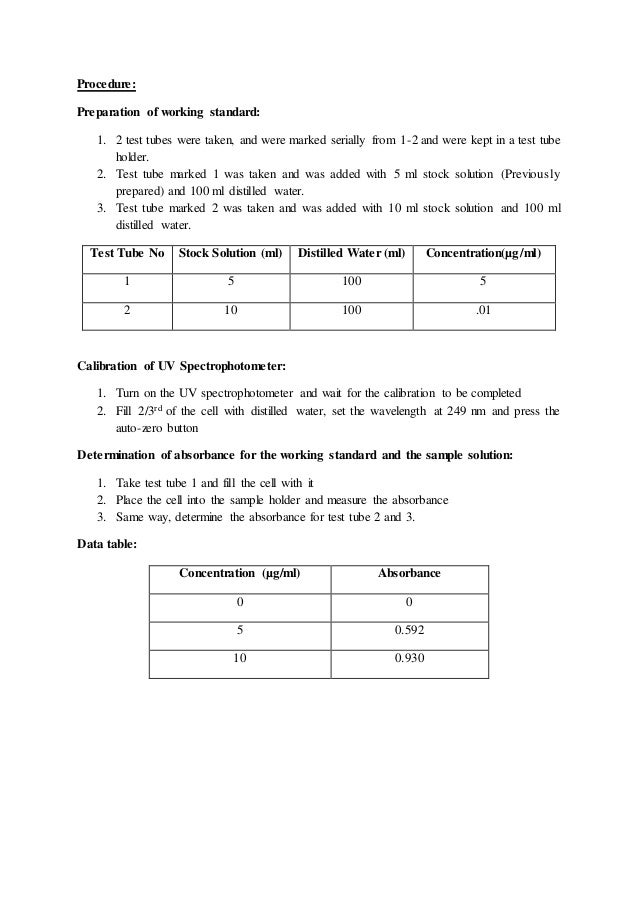
Determination Of A Wavelength Of Maximum Absorbance Lmax And

5 Colorimetric Analysis 4 Hitachi High Tech Global
Uv Visible Spectroscopy
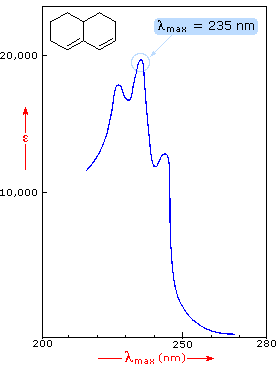
Uv Visible Spectroscopy

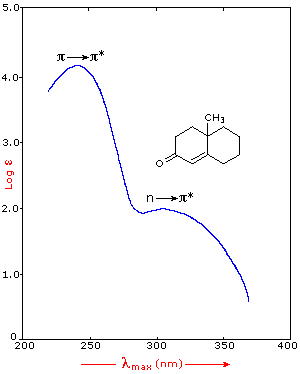
Interpreting Uv Spectra Mcc Organic Chemistry

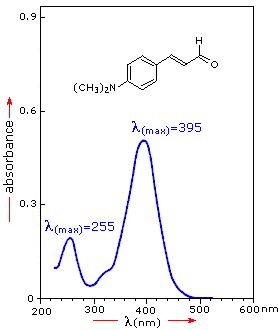
Uv Visible Spectroscopy

What Is Absorbance Absorbance Measurement Absorbance Assays Molecular Devices
