When you use a spectrophotometer you are not actually measuring the absolute absorption of the sample but the sum of all light loss between the light source and the detector. Make a whole rainbow by mixing red green and blue light.
/beers-law-definition-and-equation-608172_FINAL-20ddc4fef437472db0a0ebe395770c76.png)
Beer S Law Definition And Equation

Beer Lambert Law An Overview Sciencedirect Topics
Using Uv Visible Absorption Spectra
They are all related in through the Beer-Lambert Law.

Why are absorbance and concentration related. Y ax 2 bx c where solving for x determines the protein concentration of the sample. Citation needed Unlike other protein assays the Bradford protein assay is less susceptible to interference by various chemical compounds such as sodium potassium or even carbohydrates like sucrose that may be present in protein samples. The question arises why transmission is not directly used for.
X axis concentration should be 0 - 045M Y axis absorbance should be 0 - 15 Depending on whether you used anhydrous or pentahydrate your 00125 gmL corresponds to 005 - 008M. Why is Absorbance the Preferred Unit Over Transmittance. Answer 1 of 5.
Short answerUsing the maximum wavelength gives us the best results. The concentration is simply the moles L-1 M of the sample dissolved in the solution and the length is the length of the cuvette used for the absorbance measurement and is typically 1 cm. IR absorbance spectroscopy obeys the BeerLambert law whereby band absorbance is linearly related to concentration of the molecule in solution.
The old IR-spectra can only be found in Transmittance mode. Understand the Beer-Lambert law for absorbance A ɛ x l x c. This is different for.
The law states that the concentration of a chemical is directly proportional to the absorbance of a solutionThe relation may be used to determine the concentration of a chemical species in a solution using a colorimeter or spectrophotometer. The absorbance and transmittance are related to each other. Absorbance is directly proportional to the concentration of the molecules and is measured on a logarithmic scale from 0 to infinity.
This allows the estimation of the association constant and thereby ΔG by measuring the decrease in the intensity of the free AH band on formation of an H-bond along with knowledge of the total amounts of each substance present. So it only measures the Transmittance. Some of the more traditional methods of total protein quantitation include the measurement of UV absorbance at 280 nm A 280 Bicinchoninic acid BCA.
According to Beer-Lambert Law. Absorbance is directly proportional to concentration and length. Due to this influence H and OH- are related to the basic definitions of acids and bases.
This is because at the peak absorbance the absobance strength of light will be. The Beer-Lambert law states that there is a linear relationship between the concentration and the absorbance of the solution which enables the concentration of a solution to be calculated by measuring its. Expansion or contraction of the solvent-This may lead to a change in the concentration of the solution and affect the absorbance as absorbance is linearly related to concentration.
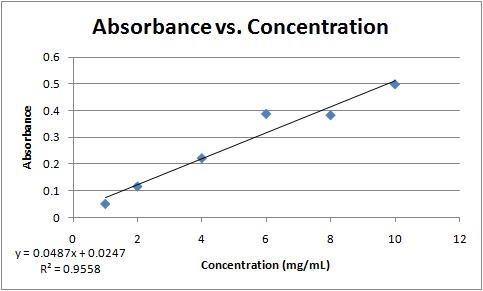
Since accurate protein quantitation is essential to all experiments related to protein studies different methods have been developed to measure the concentration of proteins in a given assay. Plotting a graph with the absorbance value as the dependent variable Y-axis and concentration as the independent variable X-axis results in an equation formatted as follows. Absorbance value to a standard curve.
The absorbance is found to be 0209 when this Solution is placed in a 100 cm cuvette and 258 nm radiations are passed through it. The main absorbance equation is the Beer-Lambert Law which is. A standard curve is where you do a serial dilution of the standard of known concentration eg.
The amount of light unable to pass through a sample is measured as the absorbance value. However enzymes become saturated when the substrate concentration is high. But after the use of microprocessors it automatically converts transmittance into absorbance.
A ε b c Where ε is the wavelength-dependent molar absorptivity coefficient with units of. ε is the molar absorptivity constant. The relation is most often used in UV-visible absorption.
Article Beers Law Why Absorbance Depends Almost Linearly on. It is the absorbance of a substance placed in 1cm cuvette cell when the concentration is 1 molar. Absorbance quantitation of DNA works on samples ranging from about 025 µgmL to about 125 µgmL in a microplate format.
More important we will show how determining either the integrated absorbance of a band or the classical oscillator strength instead of the absorbance at a certain spectral point or the peak absorbance can overcome the corresponding limitation. Beers Law is an equation that relates the attenuation of light to properties of a material. When working in concentration units of molarity the Beer-Lambert law is written as.
Light passing through a sample solution will partially be absorbed by molecules present in the sample. Schlieren effect-This effect may occur with temperature changes leading to a series of convective currents which may change the true absorbance. A Calculate the specific absorptivity including units of yeast t.
At a neutral pH of 7 pure water the concentration of both H ions and OH- ions is 107 M. The rate of a chemical reaction increases as the substrate concentration increases. The absorbance of a DNA sample measured at 260 nm on a spectrophotometer or microplate reader can be used to calculate its concentration.
Some of that light is simply not aimed directly at the detector some of it is scattered by the cuvette. The increase of absorbance at 595 nm is proportional to the amount of bound dye and thus to the amount concentration of protein present in the sample. Enzymes can greatly speed up the rate of a reaction.
ε is the wavelength-dependent molar absorbtivity coefficient and it is constant. 100 uL of 1 mgmL 100 uL buffer - mix mix mix - 100 uL of that mix to 100 uL of buffer - mix mix mix and then you measure the signal from each of those values using whatever assay youre using and then you plot that signal against concentration. The higher the H concentration the lower the pH and the higher the OH- concentration the higher the pH.
The concentration of yeast t-RNA in an aqueous solution is 10 M. By increasing the enzyme concentration the maximum reaction rate greatly increases. The standard equation for absorbance is A ɛ x l x c where A is the amount of light absorbed by the sample for a given wavelength ɛ is the molar absorptivity l is the distance that the light travels through the solution and c is the concentration of the absorbing species per unit volume.
Change the wavelength of a monochromatic beam or filter white light. In general concentration is not linear to absorbance even in the absence of interactions - only integrated absorbance is. But how are wavelength and concentration related to absorbance.
Where A is the absorbance. Calculation of absorbance A log I 0 I Modern photometers automatically convert the transmission of a sample into absorbance which is defined as the negative decadic logarithm of transmission. In the following we will briefly introduce why absorbance is not linearly dependent on the concentration even in the absence of any interactions.
View the light as a solid beam or see the individual photons.
Chem 125 Experiment Ii

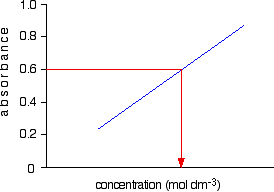
Determination Of Sample Concentration
Question 7f9f2 Socratic
Chem 125 Experiment Ii

The Absorbance Versus Concentration Graph Of Chlorpyrifos Download Scientific Diagram
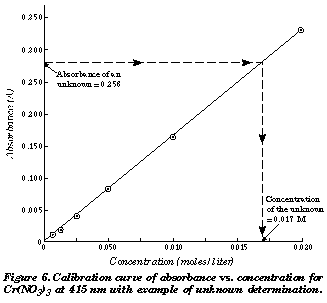
How Do You Calculate Concentration From Absorbance Socratic
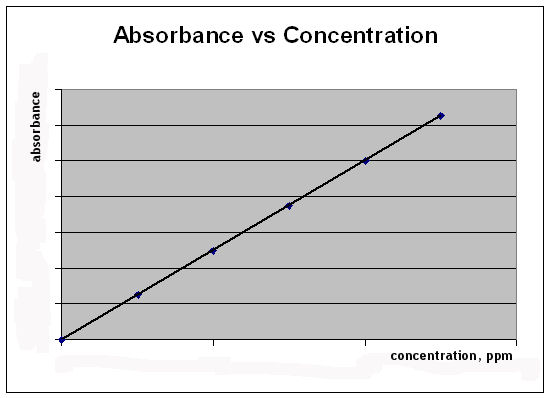
Untitled

Difference Between Calibration Curve Absorbance And Concentration Compare The Difference Between Similar Terms
